Polymers are ubiquitous in the aerospace industry, but the long-term exposure of such
materials in the Low Earth Orbit (LEO, the region up to ~1000 km from the surface, where most
satellites orbit) can lead to a systematic degradation of their properties, due to chemical erosion
from atomic O and O + ions (the main atmospheric components at such altitudes). Despite
numerous ground-based exposure simulations and flight tests, a detailed description of the
chemical erosion mechanisms is still lacking. In addition, energetic ion bombardment
extensively induces polymer surface ionization and atomic displacement, resulting in massive
structural transformations. While alternative and self-regenerative methods of protection have
been developed for organic polymeric materials used in space, a chemical attack on the most
fragile part of the polymer is still possible. In this project, we propose a novel approach to gain
insight into the mechanisms of chemical erosion due to O and O + . To do so, we have partitioned
the structure of three space-polymers (graphite, polystyrene and Kapton) into small molecular
moieties that retain the relevant chemical functions, but can be brought to the gas phase. By
running experiments under single collision conditions, we aim to elucidate the mechanisms of
chemical attack with a specific portion of the polymers. The obtained data will be of great help
to understand chemical erosion pathways and to identify the most fragile parts of the polymer
structure. This will, in turn, allow the design of more robust polymers with the elimination of the
most fragile chemical groups, possibly improving the resistance of polymers to be used in space
LEO satellites, stations and spacecrafts.
The project is a joint effort between the research group of prof. Nadia Balucani (University of
Perugia) and our team. The Perugia group will focus on the reactions of O radicals with the
selected molecules and on electronic structure calculations of the reactive potential energy
surfaces and statistical calculations of the product branching ratios. Our team will study the
reactivity of O + , known to be present in the thermosphere not only in its ground ( 4 S) but also in
the excited states ( 2 D and 2 P). Due to their long lifetimes (3.6 hours for 2 D, 4.6 s for 2 P) they have
time to react prior to de-excitation in the low-pressure conditions of LEO, and since they carry
3.3 and 5.0 eV additional energy, their reactivity is expected to be different from that of 4 S.
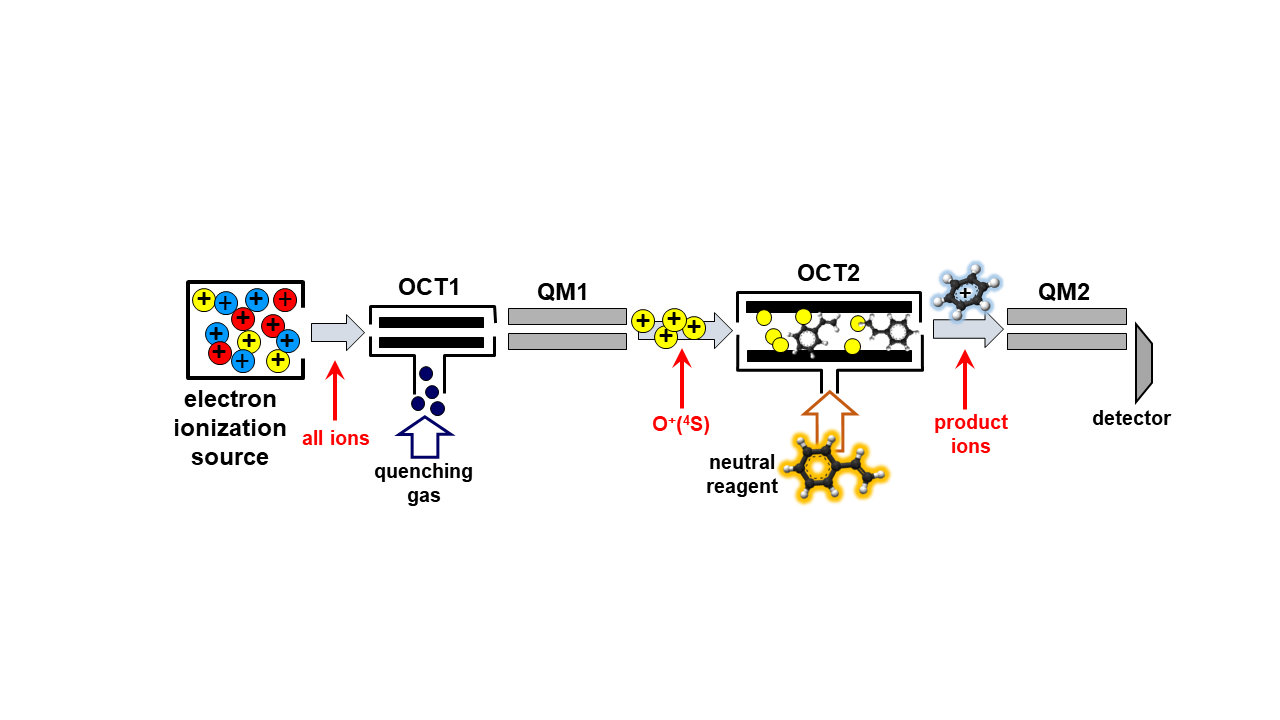
A guided ion beam mass spectrometer (GIB machine, see Figure below) will be used for the
measurements: the set-up is equipped with an electron ionization source, two RF octupoles plus
collision cells (OCT1 and OCT2) and two quadrupoles mass spectrometers (QM1 and QM2).
Mass-selected ions (via QM1) react with neutrals under single collision conditions in OCT2, and
reactant and product ions are detected via QM2. Absolute cross sections and product branching
ratios are measured as a function of the collision energy in the range from 0.1 to about 50 eV.