Satisfying the world’s increasing energy demand while reducing negative impacts on the Earth climate is a key challenge in present days. The increase in the Earth’s surface temperature is related to the accumulation in the atmosphere of greenhouse gases (GHG). Carbon dioxide and methane play a predominant role due to anthropogenic emissions. An obvious way to cut down the release of CO2 is to substitute quotas of fossil fuels with low-carbon energy resources. However, solar and wind sources fluctuate in time and can then create mismatches between supply and demand. Above a critical threshold share, variable energy sources exacerbate load matching and pose issues to their direct integration into the power grid. A primary challenge is thus the need for large-scale storage. Since it would allow decoupling supply and demand, it eventually permits increasing the renewable share in power generation. A promising storage option is a Power-to-Fuels strategy, which uses renewable electricity to produce synthetic fuels.
Converting CO2 into synthetic carbon fuels using renewable energy is a way to improve renewable energy’s transport and storage capability and simultaneously realize CO2 utilization. Synthetic fuels are an attractive option because of their high energy densities. They can also be deployed within the existing transport infrastructure and find a role in harder to electrify sectors, such as aviation.
A plasma-based technology is a promising way to convert GHG into value-added products. Plasma discharges can be far from thermal equilibrium, with the electrons’ kinetic energy much higher than those of more massive particles. Also, the vibrational temperature may be higher than the gas temperature. This peculiar mean-energy hierarchy can be usefully exploited to drive chemical reactions with minimized thermal energy losses. Eventually, the plasma technology has some significant advantages: it can operate at room temperature and atmospheric pressure; it is highly flexible regarding input flows and on/off switch times, which are very short. This flexibility makes plasmolysis particularly suitable for the storage of electricity, managing production peaks, and stabilizing the distribution grid. Reactors have low investment and management costs and are scalable in size and to different applications. Finally, the plasma approach does not use rare or expensive materials, an almost unique feature in the panorama of the possible technologies proposed.
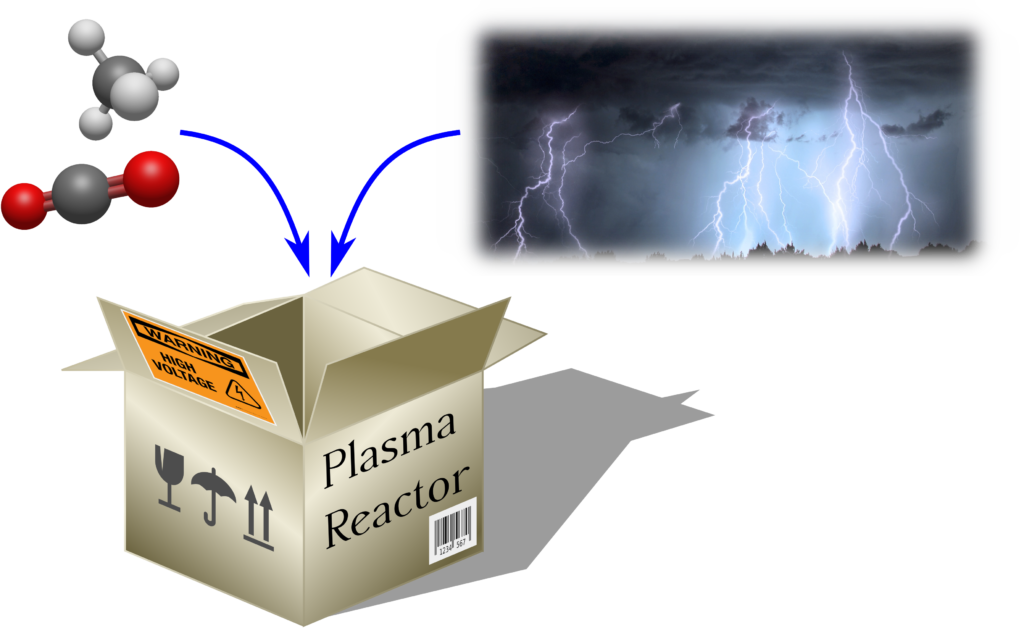
We study the nanosecond repetitively pulsed (NRP) discharge, a particular electrical discharge characterized by very short voltage pulses (around 10 ns). The repetition rate of the pulses plays a crucial role in the evolution of the process. For this reason, we have assessed the influence of the pulsing scheme in an NRP discharge for two different mixtures of reagents: only CO2 (link to the paper) and CO2+CH4 (dry reforming of methane, link to the paper).